PRODUCT ANALYSIS
Testing the liposomal market
As a rule, food supplement companies are fairly private about their ingredient sources and production practices.
Moreover, many make efficacy claims that have little to no scientific proof to back them up, despite being emblazoned with marketing phrases such as
“backed by science.”
As recently as November 2021, the ASA published a ruling on the marketing of liposomal supplements, following the analysis of a particular brand of “liposomal” Vitamin C.
This brand’s products were found to be devoid of any liposomes, despite having been heavily marketed as such since its launch in 2018. “Because we had not seen conclusive evidence confirming the presence of liposomes in [brand x] product, we concluded that the claims “liposomal vitamin C”, and similar claims about the liposomal nature of the product, had not been substantiated and were therefore misleading.”
We already knew this.
lipolife have been analysing commercial liposomal Vitamin C products for the past seven years.
To date, we have only found one other product on the market which displays characteristics of a true, quality liposomal formula.
Believe it or not, there is currently no standardisation or specific labelling requirements for liposomal manufacturers. As a consumer, it can be difficult to know what you are actually getting.
Even the terminology can be misleading. The word “liposomal” does not mean “liposome,” these terms are not one and the same even though they do sound similar. The term liposomal is widely being used simply to mean “containing fat”. So products containing just fat (lipid) and vitamin C mixed together are being termed as liposomal but without the presence of liposomes.
It’s a minefield.
Asking the right questions and validating these processes with the manufacturer is a good first step in understanding a quality product. Creating effective liposomal delivery in food supplements is an exact science. The process and technology is complicated and involves intricate steps to ensure proper stability, potency, compatibility and consistency.
Below you will find the results of our liposomal characterisation analysis to give you, the consumer, an insight into the quality of liposomal products freely sold across the UK and Europe.
Liposomal Characterisation
- Particle Size
- Polydispersity Index
- Nutrient Assay
- pH
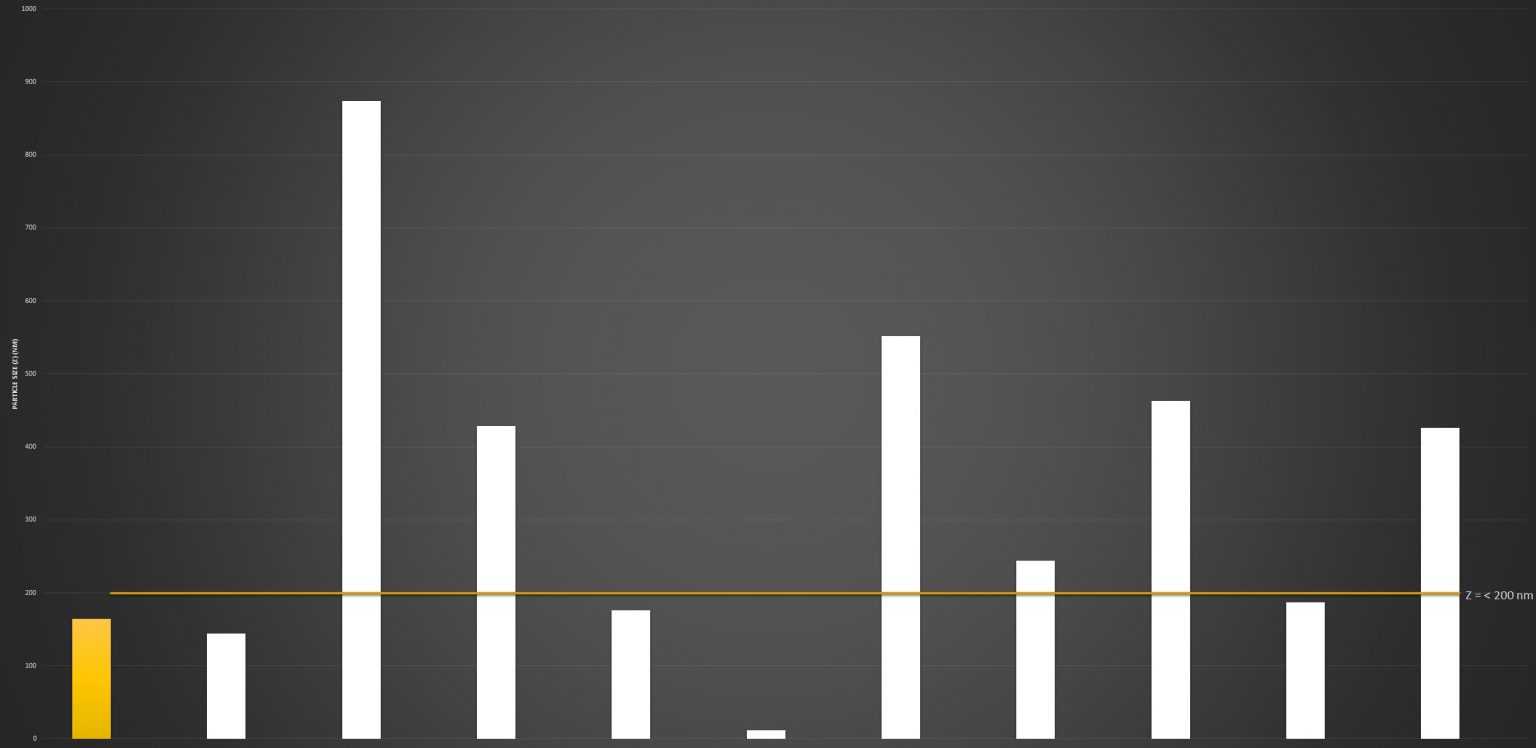
Particle Size
In order for liposomes to be effectively and efficiently absorbed into the cells, they need to be a certain size. If the liposome matrix is too large, it will not be absorbed and will instead be excreted. Ideally for liposomal Vitamin C, the particle size should be between 100nm and 200nm.
The above is a snapshot of the particle sizes from ten liposomal Vitamin C products alongside lipolife LVC2, highlighted.
The standard bar indicates a size of 200nm. You will note that some products fall within the range considered optimum for absorption. Several products contained liposomes over 400nm and one product completely lacked any liposomes, returning a false positive from a molecule of glycerol.
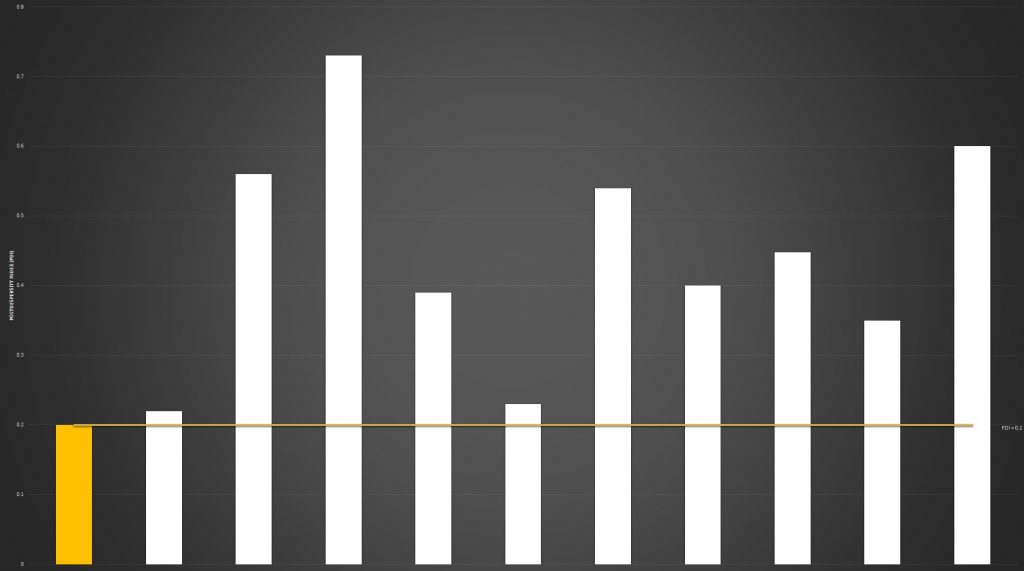
Size Index
The polydispersity index (PDI) is a measure of the variability of a sample based on size. The numerical value of PDI ranges from 0.0 to 1.0. A value of 0.0 indicates a sample containing perfectly uniform sized particles. A PDI of 1.0 indicates a sample containing multiple particles of varying size and shape.
Quality, lipid-based food supplements, such as liposome and nanoliposome formulations, should present a PDI of 0.2 to 0.3, ideally ≥0.2.
In short, a low PDI means a stable formula with higher absorption potential.
A high PDI value will result in an unstable formula with lower absorption capability.
Our PDI analysis once again showed a significant difference in quality across the products tested.
lipolife LVC2 returned a PDI of 0.2, with only two other brands returning similar results. Those brands which were found to have a PDI above 0.7 indicate formulas that are unlikely to be manufactured to a high quality.
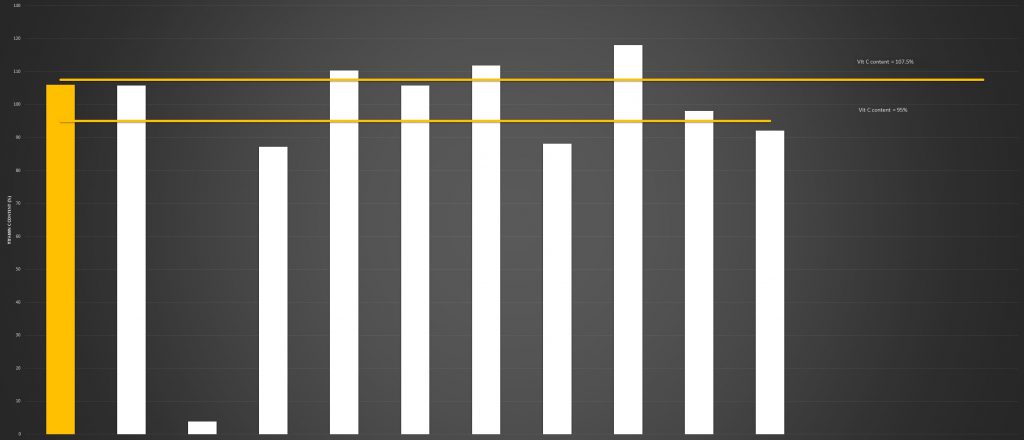
Nutrient Assay
In 2019, Which published an article following an investigation into food supplements. Staggeringly, of the eight supplements they tested, three of the biggest names in the industry were marketing products that did not contain the stated dosage of nutrient.
- Vitabiotics Wellwoman Original contained only 65% of the declared vitamin D: 3.2mcg (micrograms) instead of the 5mcg listed on the label.
- Holland & Barrett ABC Plus multivitamins contained 362mcg of vitamin A instead of the advertised 692mcg (only 52% of what’s stated).
- Nature’s Best Multiguard Balance supplements claimed to contain 10mg (milligrams) of vitamin B6 per tablet, but [Which] tests found negligible amounts.
By law, supplements can contain up to 50% more or 20% less vitamin, and 45% more and 20% less mineral than what is stated on the label.
When testing the Vitamin C content of the liposomal products were purchased, the majority fell into the acceptable parameters of the stated dose.
One particular product, a product which sells very well on a popular ecommerce platform,
contained less than 4% of the stated Vitamin C content.
This same product had a pH of 2.87, a particle size of 873.60nm and a PDI of 0.56. This is far removed from a quality liposomal supplement.
How to identify a quality liposomal supplement
Let us do it for you!
If there is a liposomal supplement on the market which you have purchased and found the quality to be concerning, email us. We will purchase the product in question and run it through our liposomal characterisation tests.
The results will be published on our website, omitting the brand information, and we will also email you our conclusion.