Are all liposomal supplements created equal?
Believe it or not, there is currently no standardisation or specific labelling requirements for liposomal manufacturers. This means, as a consumer, it can be difficult to know what you are actually buying.
Even the terminology can be misleading.
The word “liposomal” does not mean “liposome.” These terms are not one and the same, even though they sound similar. The term liposomal is widely being used simply to mean “containing fat”. So products containing just fat (lipid) and vitamin C mixed together are being termed as liposomal but without the presence of liposomes.
Identifying authentic liposomal supplements.
We have been analysing commercial liposomal supplements for the past seven years. To date, we have only found one other product on the market which displays most characteristics of a true, quality liposomal formula.
As recently as November 2021, the Advertising Standards Agency published a ruling on the marketing of liposomal supplements, following the analysis of a particular brand of “liposomal” Vitamin C.
This brand’s products were found to be devoid of any liposomes, despite having been heavily marketed as such since its launch in 2018. “Because we had not seen conclusive evidence confirming the presence of liposomes in [brand x] product, we concluded that the claims “liposomal vitamin C”, and similar claims about the liposomal nature of the product, had not been substantiated and were therefore misleading.”
With so many liposomal brands coming to market making efficacy claims that have little to no scientific proof to back them up, how can you identify an authentic liposomal supplement?
Asking the right questions is a good first step in understanding a quality product. Creating effective liposomal delivery in food supplements is an exact science. The process and technology is complicated and involves intricate steps to ensure proper stability, potency, compatibility and consistency.
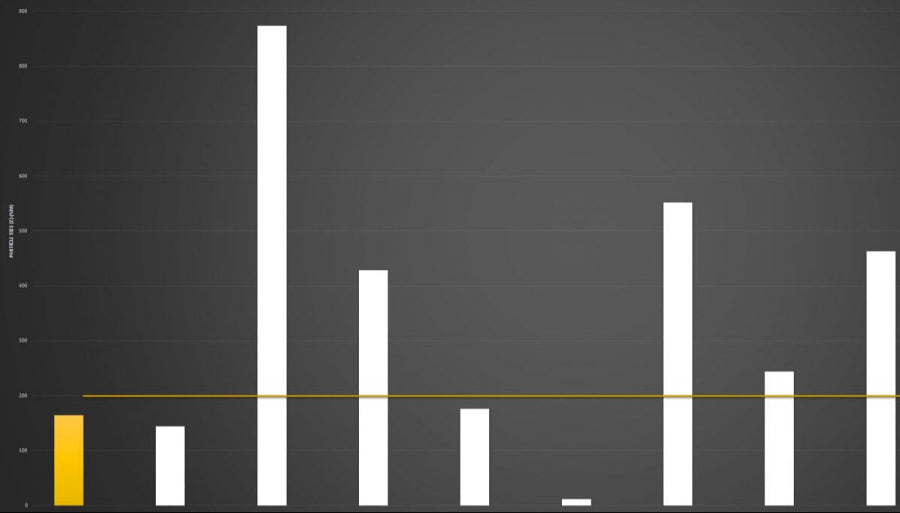
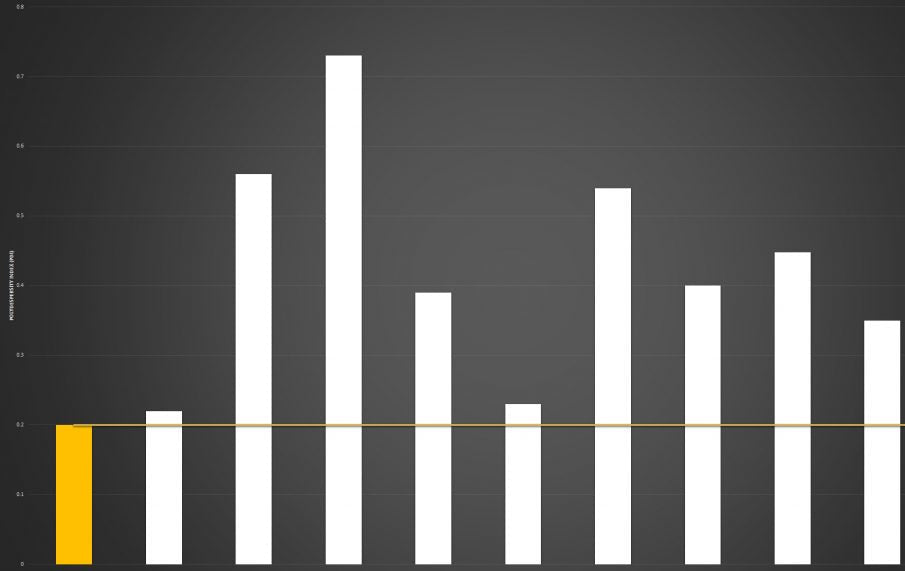
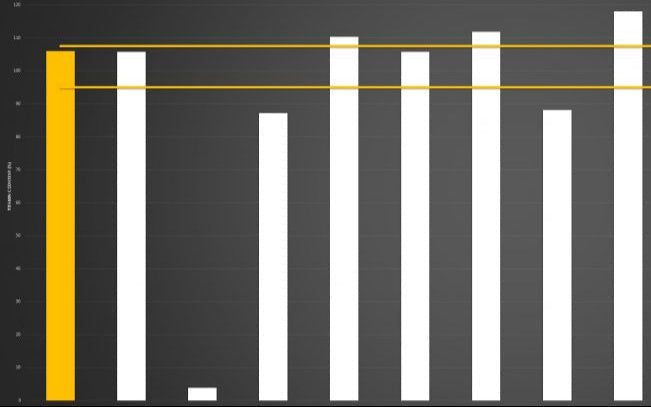
Below you can review the results of our liposomal characterisation analysis. Our aim is to give you, the consumer, an insight into the quality of liposomal products sold across the UK and Europe.
Marketing v Science
If you're researching the liposomal supplement market, you will have seen brands boasting claims like “10x absorption” or “95% absorption.”
Here’s the reality: absorption is not a fixed number - it is a variable dependent on the individual.
Rather than relying on misleading marketing statistics, which are seemingly plucked from obscurity, lipolife focus on the proven science of liposomal delivery and the meticulous quality control measures that ensure our liposomes are truly effective.
Our team of expert scientists have reviewed some of these claims made by liposomal brands.
A lot of the claims made are unsubstantiated, with little or no supportive scientific evidence.
Fact or Fiction?
Our mission - identify myths and misinformation and provide solid scientific “debunking” so you, the consumer, can separate science fact from science fiction.
Thicker consistency means more liposomes.
Thicker consistency means more liposomes.
Fiction!
Viscosity (thickness) is a physical characteristic of liquids. There are many factors effecting a liquid’s thickness such as the use of polymers, gelation agents or solid materials that absorb water such as starch in soup.
A generalisation of “thickness” therefore, cannot be used as an indication of a high concentration of liposomes.
You should have equal proportions of nutrients to phospholipids.
You should have equal proportions of nutrients to phospholipids.
Fiction!
If the aim is the nutritional value of both ingredients, there is no evidence suggesting each have their own required portions in the normal diet.
If the aim is formulation stability, the ratio of raw materials are formulated by experts based on their studies of the optimal proportions for a perfect formulation, both in terms of stability and palatability.
A watery product suggests there are not enough phospholipids.
A watery product suggests there are not enough phospholipids.
... or that the quality of the phospholipids is not up to standard for liposomes.
Fiction!
Ingredient proportions are formulated by experts based on extensive research and development into optimal ratios for a perfect formulation.
A generalisation of “thickness” therefore, cannot be used as an indication of a high concentration of liposomes or indeed a simple quality assay.
Our liposomes are digested within 4 minutes.
Our liposomes are digested within 4 minutes.
Simply impossible!
Transit time through the stomach is 1-2 hrs, at which point the nutrient reaches the intestine where absorption takes place. Once absorbed, the nutrient is released and reaches systemic circulation to exert its therapeutic effect.
From an efficacy view-point, it is more important for the nutrient to be absorbed in sufficient concentration rather than being digested. The degradation of nutrients by digestion or first-pass effect, before reaching systemic circulation, leads towards loss of action and sub-therapeutic effects.
Avoid plastic bottles due to leaching.
Avoid plastic bottles due to leaching.
Fiction!
The process of chemical migration/ leaching of plastics into food is dependent on the type of plastic used, contact time, temperature, food type, and the contact area between the plastic container and the food. Therefore, whether your plastic container is safe to reuse depends on what it was designed for and how you are using it.
We use PET bottles, and there are no chemical phthalates or bisphenol A (BPA) in PET plastic. PET plastic does not leach these substances. PET plastic is approved as safe for food and beverage contact by the FDA and similar regulatory agencies throughout the world, and has been for more than 30 years
Dry Liposomes: Debunking Misinformation with Real Science
In recent months, we’ve observed a concerning rise in misinformation about powdered (dry) liposomes, particularly within the food supplement industry.
Several contract manufacturers, often lacking true expertise in nanoencapsulation, have made claims such as:
- “Only liquid liposomes preserve full integrity and function.”
- “Turning liposomes into powder destroys their structure.”
- “Liquid liposomes are the only ‘real’ liposomal delivery system.”
These statements are not only misleading, they are scientifically inaccurate.
Our Scientists
The experts behind the encapsulation.
With over 35 years of combined expertise in liposomal encapsulation, the team behind lipolife® includes formulation scientists, researchers, medical doctors and PhDs, all dedicated to one goal: developing highly absorbable, clinically reliable supplements.
From raw material selection to finished product, we apply rigorous quality control at every stage, ensuring consistency, purity and performance.

Professor Mohammad Najlah
BPharm, PgDip, PhD, FHEA, FRSC
Mohammad Najlah has been at the forefront of nano-encapsulation for over two decades.
He is a Professor of Pharmaceutics and Nanomedicine and Lead of the Pharmaceutical Research Group, part of Anglia Ruskin University’s Medical Technology Research Centre (MTRC). Mohammad obtained his PhD from the University of Manchester, focusing on the development of nano-medicines based on dendrimer prodrugs.
Professor Najlah was instrumental in developing ARU’s SuperLabs Complex, the establishment of a new undergraduate and postgraduate programmes and leading the Pharmaceutical Research Group.

Dr. Hanan Abdalmaula
M.Pharm.Ph.D.
As the Lead Formulation Scientist and Head of R&D, Dr. Abdalmaula's role involves driving innovation, overseeing product development and ensuring regulatory compliance. Dr. Abdalmaula oversees the entire product development process from concept through to scale-up and production.